At proposal submission and award the PI needs to declare conflicts of interest and nepotism. You will find information about nepotism and COI, along with required forms, on the Provost’s Resource page – Forms, Memos, and Guidelines. For additional guidance regarding Individual Conflicts of Interest Policy, view Section 5.10 of the Faculty Handbook.
For proposal routing, the following items are needed, as applicable
Disclosure Form | Review Form (see Section 5.10 of the Faculty Handbook)
The COI Management Plan should contain the following:
For example:
Example 2:
Associate Dean Z will review and approve the subcontract statement of work and budget and will have signature authority for expenditures on the award for any salary or travel support of Professor X. Associate Dean Z will also review and approve the FUA terms, in collaboration with the Office of Research Services.
Conflicts of interest in research may occur when outside financial interests or commitments/relationships compromise, or have the appearance of compromising, the professional judgment of a researcher when designing, conducting, or reporting research. If an investigator believes a financial or commitment conflict of interest exists with a proposed project for themselves or a member of their team, then they are required to disclose the conflict and follow the guidance below. Even if there is no identified PHS FCOI, the Investigator should ensure compliance with the University Conflict of Interest Policy (https://apps.hr.ou.edu/FacultyHandbook/#5.10).
Conflicts of interest in research may occur when outside financial interests or commitments/relationships compromise, or have the appearance of compromising, the professional judgment of a researcher when designing, conducting, or reporting research. If an investigator believes a financial or commitment conflict of interest exists with a proposed project for themselves or a member of their team then they are required to disclose the conflict and follow the guidance below. Even if there is no identified PHS FCOI, the Investigator should ensure compliance with the University Conflict of Interest Policy (https://apps.hr.ou.edu/FacultyHandbook/#5.10).
In 2011, the Public Health Service (PHS) released revised financial conflict of interest (FCOI) regulations (42 CFR 50) that apply to any institution receiving funds from a PHS entity. The University’s PHS FCOI policy and the implementation plan correspond with the mandate of this new regulation. Note that these requirements affect not only National Institute of Health (NIH) proposals/awards but other agencies (listed below) who have adopted this guidance.
Disclosure Requirement
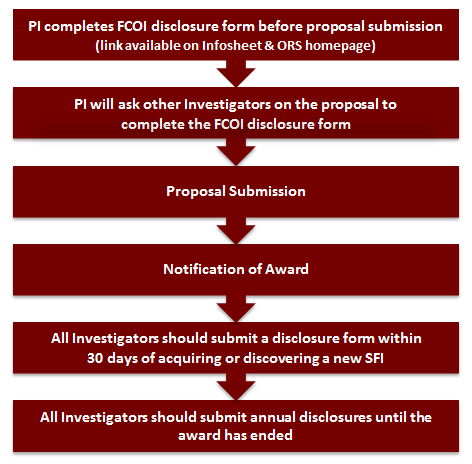
Disclosure of PHS FCOI (and FCOI) is done by the Primary Investigator (PI) (see definition below) at submission of the information sheet prior to proposal submission. According to the federal reporting requirements, the project director or investigator and any other person, regardless of title or position, who is responsible for the design, conduct, or reporting of research must submit a FCOI disclosure form at the time of application, within 30 days of acquiring or discovering a new Significant Financial Interest (SFI), and annually after the Notice of Award. If there is insufficient space to include all your SFIs in one report, submit a second report with those relationships that did not fit in the first submission.
Training Requirement
According to the federal requirements, all PHS-funded 'Investigators' at the University must complete the online FCOI training available here: https://www.citiprogram.org/. Successful completion of the training is required prior to the expenditure of funds on any newly funded projects, including noncompeting continuation awards. This applies to all PHS-sponsored research projects as of August 24, 2012. Training must be completed at least every four years. Current PHS awards are not subject to these new requirements until the noncompeting continuation is awarded.
New users to the CITI program must create an account. The same CITI account can be used for both the FCOI requirement and the University's IRB training requirement.
In addition, training is required immediately when:
Definitions and PHS Agencies/Delegations
Investigator, for the purposes of this policy, means the Principal Investigator and any person listed by the Principal Investigator as responsible for the design, conduct, or reporting of their sponsored program(s). These individuals are listed at the time of proposal submission.
Normally, all senior research personnel should be listed as Investigators. All of the following should be considered, to the extent they are responsible for the design, conduct, or reporting of the sponsored program: professorial faculty, research associates, emeritus faculty, research collaborators, visiting scientists, postdocs, GRAs, or students.
Significant Financial Interest (SFI)
SFI means a financial interest consisting of one or more of the following interests of the Investigator (and those of the Investigator's spouse/domestic partner or dependent children) that reasonably appear to be related to the Investigator's University responsibilities:
· With regard to any publicly traded entity, a significant financial interest exists if the value of any remuneration received from the entity in the twelve months preceding the disclosure and the value of any equity interest in the entity as of the date of the disclosure, when aggregated, exceeds $5,000.
A FCOI means an SFI that could directly and significantly affect the design, conduct, or reporting of PHS-funded research.
[1] The requirements regarding Investigator financial conflicts of interest in research funded under PHS grants or cooperative agreements can be found at http://grants.nih.gov/grants/FCOI_Final_Rule_inspection_Desk.pdf, pp 53283-53288.
Public Health Service (PHS) means the Public Health Service of the U.S. Department of Health and Human Services and any components of the PHS to which the authority of the PHS may be delegated. The components of the PHS include, but are not limited to:
Other agencies following PHS regulations include (but aren’t limited to):
Alliance for Lupus Research, Alpha-1 Foundation, American Asthma Foundations, American Cancer Society, American Heart Association, American Lung Association, Arthritis Foundation, CurePSP, JDRF - Juvenile Diabetes Research Foundation, Lupus Foundation of America, Patient-Centered Outcomes Research Institute (PCORI), and Susan G. Komen Foundation
Institutional Responsibility to Review Disclosures
Upon receipt of the Information Sheet (where SFI/FCOI is identified) and/or when the official disclosure process detailed above is initiated and prior to award, the internal process for PHS FCOI/COI begins (see above). Upon receipt of award from the Public Health Service and prior to expenditure of any funds, as well as within 60 days for any interest that the University identifies as conflicting subsequent to the University’s initial report under the award, or at the time of an NIH award extension, the University must determine:
As part of the University’s responsibilities, the Office of Research Services coordinates with designated officials to ensure the certification documents, management plan, and any required training is completed prior to finalizing award processing and within regulation time requirements for FCOI disclosed after initial award set up.
Reporting FCOI
The University will monitor investigator compliance and report identified FCOI to the NIH via eRA Commons FCOI Module per the regulation. Any investigator who has knowledge or suspicion of an FCOI that has not been reported should contact the chair of the Conflict of Interest committee or email VPRFCOI@ou.edu
Designation of Institutional Official and Requirements
The Provost has established a Conflict of Interest committee. The Conflict of Interest committee will handle review of the Investigators’ FCOI Disclosure forms as required by this policy and pursuant to the faculty handbook and/or as required by the Board of Regents policy.
Subrecipient Requirements
If sub-awardees/subrecipients, contractors, or collaborators carry out agency funded research, the University will take reasonable steps, via written agreement, to ensure that the collaborating entity has its own policies in place that meet the requirements of the PHS policy or that investigators working for such entities follow the policies of the University.
Situations of Noncompliance
In the event the University identifies a significant financial interest that was not disclosed in a timely fashion by the Investigator or was not previously reviewed by the University during an on-going PHS funded research project, the University will, within 60 days, determine whether a financial conflict of interest exists and will implement a management plan.
In addition, whenever a financial conflict of interest is not identified or managed in a timely manner, the University will, within 120 days of its determination of non-compliance, complete a retrospective review of the investigator’s activities and the PHS funded research project. The purpose of the review is to determine whether any PHS research conducted during the period of non-compliance was biased in its design, conduct, or reporting.
The documentation of the retrospective review will include all the elements of a regular review (Proposal/Project number, Title, PD/PI name [or contact if multiple PI/PD model used], name of investigator with conflict; name of entity with which the Investigator has an FCOI), and the reasons for the retrospective review. The methodology of the review and findings/conclusions of the review will also be documented.
Based on the retrospective review, if appropriate, the University will update the previously submitted FCOI report, specifying the actions that will be taken to manage the FCOI and monitor Investigator compliance with the plan. If bias is found, the University will notify the PHS Awarding Component promptly and submit a mitigation report to the PHS Awarding Component. The mitigation report will include the key elements documented in the retrospective review, the impact of the bias on the research project and University’s plan of action or actions to eliminate or mitigate the effect of the bias.
In any case in which Health and Human Services (HHS) determines that a PHS-funded project of clinical research whose purpose is to evaluate the safety or effectiveness or a drug, medical device, or treatment has been designed, conducted or reported by an Investigator with a financial conflict of interest that was not managed or reported as required by PHS regulations, the University will require the Investigator involved to disclose the financial conflict of interest in each public presentation of the results of the research and to request an addendum to previously published presentations.
Public Accessibility
Prior to the expenditure of funds, the University will make certain that information concerning FCOI held by Senior/Key personnel is publicly accessible by a written response to any requester within five business days of a request or as required by law. This response will provide current information (subject to annual updates and within 60 days of identification of a new FCOI) and will include: the Investigator’s name, title, and role with respect to the research project; the name of the entity in which significant financial interest is held; the nature of the significant financial interest; and the approximate dollar value of the significant financial interest (in pre-specified dollar ranges), or a statement that a value cannot be readily determined.
If the University responds to written requests for the purposes of this subsection, the University will note in its written response that the information provided is current as of the date of the correspondence and is subject to updates, on at least an annual basis and within 60 days of the University's identification of a new financial conflict of interest, which should be requested subsequently by the requestor.
Maintenance of Records
The University will maintain records relating to all Investigator disclosures of financial interests and the University’s review of and actions taken related to such disclosures for at least three years from the date the final expenditures report is submitted to the sponsor, or where applicable, from other dates specified in PHS regulations. Primary repository of records is the Cayuse system.
Questions
Inquiries about the NIH FCOI regulation for grants and cooperative agreements may be directed to: VPRFCOI@ou.edu.
The Department of Energy has issued an interim conflict of interest (COI) policy, modeled on the existing Public Health Service (PHS) financial conflict of interest regulation, that addresses both financial and organizational conflicts of interest, which will be incorporated into and made enforceable through the Special Terms and Conditions for DOE financial assistance awards. The DOE Interim COI Policy applies to all DOE-funded financial assistance awards issued on or after June 18, 2022.
1. Purpose
This University of Oklahoma (“OU”) interim Department of Energy (“DOE”) Conflicts of Interest (“COI") policy (hereinafter referred to as “Policy”) establishes standards that provide a reasonable expectation that the design, conduct, and reporting of projects wholly or in part funded under DOE financial assistance awards (e.g., a grant, cooperative agreement, or technology investment agreement) will be free from bias resulting from financial conflicts of interest.
2. Applicability
This Policy applies to each Investigator at OU who is planning to participate in or is participating in the project funded wholly or in part under the DOE financial assistance award. This Policy requires disclosure in writing of any potential conflict of interest to the DOE or pass-through entity. This Policy does not apply to Office of Indian Energy and Phase I Small Business Innovation Research (SBIR)/Small Business Technology Transfer (STTR) applications and awards. Reference to DOE includes both DOE and the National Nuclear Security Administration (NNSA).
3. Definitions
Award, financial assistance award or Federal award means the same as the definition provided in 2 CFR 200.1 for Federal award.
Contracting Activity means an organizational element that has the authority to award and administer contracting and financial assistance instruments.
Disclosure of significant financial interests means an individual’s disclosure of significant financial interests to a non-Federal entity.
DOE means the U.S. Department of Energy, the National Nuclear Security Administration (NNSA), and any components of the DOE to which the authority involved may be delegated.
DOE program office means the organizational unit of DOE, led by an officer of the Department who has been appointed by the President by and with the advice and consent of the Senate, that funds and/or manages the awards subject to this Policy. For purposes of this Policy, the term DOE program office includes the organization responsible for executing program management functions; the cognizant contracting activity; and the field elements in safety and health, administrative, management, and technical areas.
Financial conflict of interest (FCOI) means a situation in which an Investigator or the Investigator’s spouse or dependent children has a significant financial interest or financial relationship that could directly and significantly affect the design, conduct, reporting or funding of a project.
FCOI report means a non-Federal entity's report of a financial conflict of interest to the DOE program office.
Financial interest means anything of monetary value, whether or not the value is readily ascertainable.
Institution of Higher Education means the same as the definition provided at 20 U.S.C. § 1001(a).
Investigator means the principal Investigator (PI) and any other person, regardless of title or position, who is responsible for the purpose, design, conduct, or reporting of a project funded by DOE or proposed for funding by DOE. DOE program offices have the discretion to expand the definition to include also any person who participates in the purpose, design, conduct, or reporting of a project funded by DOE or proposed for funding by DOE. Such expansion will be specified in the applicable funding opportunity announcement and/or terms and conditions of the financial assistance award.
Investigator’s non-Federal entity responsibilities means an Investigator's professional responsibilities on behalf of the non-Federal entity, and as defined by the non-Federal entity in its policy on financial conflicts of interest, which may include: activities such as research, research consultation, teaching, professional practice, institutional committee memberships, and service on panels such as Institutional Review Boards or Data and Safety Monitoring Boards.
Manage means taking action to address a financial conflict of interest, which can include mitigating or eliminating the conflict of interest, to ensure, to the extent possible, that the purpose, design, conduct, and reporting of a project will be free from bias.
Non-Federal entity means OU, a State, local government, Indian tribe, Institution of Higher Education, nonprofit organization, or for-profit organization that carries out a DOE award as a recipient or subrecipient.
Non-Federal entity’s designated official means the individual designated by the non-Federal entity with the authority and responsibility to act on behalf of the non-Federal entity to ensure compliance with the Policy.
Potential conflict of interest exists when an impartial observer reasonably believes that actual or apparent past, present, or currently planned interests could constitute a conflict of interest with a project funded under a DOE award.
Principal Investigator (PI) means a principal investigator of a project funded under a DOE financial assistance award; PI is included in the definitions of senior/key personnel and Investigator.
Project means the interdependent activities funded wholly or in part under the DOE financial assistance award. A project has a defined start and end point with objectives described in an application or in an approved scope that, when attained, signify completion and achievement of a specific goal, and creation of a unique product, service, or result. For awards that include recipient cost share as part of the approved budget, activities funded with that recipient cost share are included.
Recipient means an entity, usually but not limited to non-Federal entities, that receives a Federal award directly from a Federal awarding agency. The term recipient does not include subrecipients or individuals that are beneficiaries of the award.
Senior/key personnel means the PI; any other person who significantly influences the design, conduct, or reporting of a project funded under a DOE award; and any other person identified as senior/key personnel by the non-Federal entity in the application for financial assistance, approved budget, progress report, or any other report submitted to the DOE by the non-Federal entity under this Policy.
Significant financial interest means:
(1) A financial interest consisting of one or more of the following interests of the Investigator (and those of the Investigator's spouse and dependent children) that reasonably appears to be related to the Investigator's non-Federal entity responsibilities:
a. With regard to any foreign or domestic publicly traded entity, a significant financial interest exists if the value of any remuneration received from the entity in the twelve months preceding the disclosure and the value of any equity interest in the entity as of the date of disclosure, when aggregated, exceeds $5,000. For purposes of this definition, remuneration includes salary and any payment for services not otherwise identified as salary (e.g., consulting fees, honoraria, paid authorship); equity interest includes any stock, stock option, or other ownership interest, as determined through reference to public prices or other reasonable measures of fair market value;
b. With regard to any foreign or domestic non-publicly traded entity, a significant financial interest exists if the value of any remuneration, not otherwise disclosed as current, pending, or other support, received from the entity in the twelve months preceding the disclosure, when aggregated, exceeds $5,000, or when the Investigator (or the Investigator's spouse or dependent children) holds any equity interest (e.g., stock, stock option, or other ownership interest);
c. (iii) Intellectual property rights and interests (e.g., patents, copyrights), upon receipt of income related to such rights and interests.
(2) Investigators also must disclose the occurrence of any reimbursed or sponsored travel (i.e., that which is paid on behalf of the Investigator and not reimbursed to the Investigator so that the exact monetary value may not be readily available) related to their institutional responsibilities that is not otherwise disclosed in current and pending or other support disclosures, provided that this disclosure requirement does not apply to travel that is reimbursed or sponsored by a Federal, state, or local government agency of the United States; a domestic Institution of Higher Education; or a domestic research institute that is affiliated with a domestic Institution of Higher Education. All investigators or others required to complete the DOE FCOI will specify the details of this disclosure, which will include, at a minimum, the purpose of the trip, the identity of the sponsor/organizer, the destination, and the duration. In accordance with this policy, the non-Federal designated official(s) will determine if further information is needed, including a determination or disclosure of monetary value, in order to determine whether the travel constitutes a FCOI with the project funded under the DOE award.
(3) The term significant financial interest does not include the following types of financial interests: salary, royalties, or other remuneration paid by non-Federal entity to the Investigator if the Investigator is currently employed or otherwise appointed by non-Federal entity, including intellectual property rights assigned to the non-Federal entity and agreements to share in royalties related to such rights; any ownership interest in the non-Federal entity held by the Investigator, if the non-Federal entity is a commercial or for-profit organization; income from investment vehicles, such as mutual funds and retirement accounts, as long as the Investigator does not directly control the investment decisions made in these vehicles; income from seminars, lectures, or teaching engagements sponsored by a Federal, state, or local government agency of the United States, a domestic Institution of Higher Education, or a domestic research institute that is affiliated with a domestic Institution of Higher Education; or income from service on advisory committees or review panels for a Federal, state, or local government agency of the United States, a domestic Institution of Higher Education, or a domestic research institute that is affiliated with a domestic Institution of Higher Education.
Small Business Innovation Research (SBIR) Program and Small Business Technology Transfer (STTR) Program mean the extramural research programs for small businesses that are run by the DOE Office of Science and the Advanced Research Projects Agency-Energy and certain other Federal agencies under Public Law 97-219, the Small Business Innovation Development Act, as amended, and Public Law 102-564.
Subrecipient means an entity, usually but not limited to non-Federal entities, that receives a subaward from a pass-through entity to carry out part of a Federal award, but does not include an individual that is a beneficiary of such award. A subrecipient may also be a recipient of other Federal awards directly from a Federal awarding agency.
4. Requirements
a. If an Investigator indicates on the ORS Information Sheet that a proposal is being submitted to DOE, they (as well as all other Investigators) will be directed to complete a COI Disclosure form. Each Investigator who is planning to participate in the DOE award shall disclose to the non-Federal designated official(s) the Investigator’s significant financial interests (and those of the Investigator’s spouse and dependent children) no later than the time of application for the DOE award. In the event a non-Federal entity seeks to add an Investigator after the time of application, the non-Federal entity must require the Investigator make such disclosures prior to participating in a project funded under a DOE award.
b. Each Investigator who is participating in the DOE award shall submit an updated disclosure of significant financial interests at least annually, in accordance with the specific time period prescribed by the non-Federal entity, during the period of the award. Such disclosure shall include any information that was not disclosed initially to the non-Federal entity pursuant to paragraph 4(a) of this section, or in a subsequent disclosure of significant financial interests (e.g., any financial conflict of interest identified on a DOE award that was transferred from another non-Federal entity), and shall include updated information regarding any previously disclosed significant financial interest (e.g., the updated value of a previously disclosed equity interest).
c. Each Investigator who is participating in the DOE award shall submit an updated disclosure of significant financial interests within thirty days of discovering or acquiring (e.g., through purchase, marriage, or inheritance) a new significant financial interest.
d. Each disclosure and updated disclosure must be signed and dated by the Investigator and include a certification statement that reads:
a. “I understand that this Disclosure is required to obtain funding from the U.S. Government. I, [Full Name and Title], certify to the best of my knowledge and belief that the information contained in this Disclosure Statement is true, complete, and accurate. I understand that any false, fictitious, or fraudulent information, misrepresentations, half-truths, or omissions of any material fact, may subject me to criminal, civil or administrative penalties for fraud, false statements, false claims, or otherwise. (18 U.S.C. §§ 1001 and 287, and 31 U.S.C. 3729-3730 and 3801-3812). I further understand and agree that (1) the statements and representations made herein are material to U.S. Government’s funding decision, and (2) I have a responsibility to update the disclosures during the period of performance of the award should circumstances change which impact the responses provided above.”
e. All Investigators are required to take FCOI training through CITI prior to engaging in research projects wholly or in part funded under DOE financial assistance awards for all new awards and at least every four years while engaging in DOE-funded research.
5. Implementation, Management, and Reporting of financial conflicts of interest.
1. When a COI disclosure form is submitted (as noted further below), the non-Federal designated official(s) will determine whether an Investigator's significant financial interest is related to a project funded under a DOE award and, if so related, whether the significant financial interest is a financial conflict of interest. An Investigator’s significant financial interest is related to a project funded under a DOE award when the non-Federal entity, through its designated official(s), reasonably determines that the significant financial interest could be affected by the project, could affect the project, is in an entity whose financial interest could affect the project, or is in an entity whose financial interest could be affected by the project. A financial conflict of interest exists when the non-Federal entity, through its designated official(s), reasonably determines that the significant financial interest could directly and significantly affect the purpose, design, conduct, or reporting of the project funded under a DOE award.
2. When a financial conflict of interest is identified, the non-Federal entity shall take such actions as necessary to manage financial conflicts of interest, including any financial conflicts of a subrecipient Investigator pursuant to Paragraph 10 of this Policy. Management of an identified financial conflict of interest requires development and implementation of a management plan and, if necessary, a retrospective review and a mitigation report pursuant to Section 5(4) below. A nonexclusive list of conditions or restrictions, one or more of which might be imposed to manage a financial conflict of interest, includes:
a. Public disclosure of the financial conflict of interest (e.g., when presenting or publishing the project);
b. For projects involving human subjects, disclosure of financial conflicts of interest directly to participants;
c. Appointment of an independent monitor or oversight committee capable of taking measures to protect the purpose, design, conduct, and reporting of the project against bias resulting from the financial conflict of interest;
d. Modification of the project plan;
e. Change of personnel or personnel responsibilities, or disqualification of personnel from participation in all or a portion of the project;
f. Reduction or elimination of the financial interest (e.g., sale of an equity interest); or
g. Severance of relationship(s) that create financial conflicts of interest.
3. Whenever, in the course of an ongoing project funded under a DOE award, an Investigator who is new to participating in the project discloses a significant financial interest or an existing Investigator discloses a new significant financial interest to the non-Federal entity, the non-Federal entity designated official(s) shall, within sixty (60) days: review the disclosure; determine whether it is related to the project funded under the DOE award; determine whether a financial conflict of interest exists; and, if so, implement, on at least an interim basis, a management plan that shall specify the actions that have been, and will be, taken to manage such financial conflict of interest. Depending on the nature of the significant financial interest, the non-Federal entity may determine that additional interim measures are necessary with regard to the Investigator’s participation in the project funded under the DOE award between the date of disclosure and the completion of the non-Federal entity’s review.
4. Whenever the non-Federal entity identifies a significant financial interest that was not disclosed timely by an Investigator or, for whatever reason, was not previously reviewed by the non-Federal entity during an ongoing project funded under a DOE award (e.g., was not timely reviewed or reported by a subrecipient), the designated official(s) shall, within sixty days: review the significant financial interest; determine whether it is related to the project funded under a DOE award; determine whether a financial conflict of interest exists; and, if so:
a. Implement, on at least an interim basis, a management plan that shall specify the actions that have been and will be taken to manage such financial conflict of interest going forward;
i. In addition, whenever a financial conflict of interest is not identified or managed in a timely manner, including failure by the Investigator to disclose a significant financial interest that is determined by the non-Federal entity to constitute a financial conflict of interest; failure by the non-Federal entity to review or manage such a financial conflict of interest; or failure by the Investigator to comply with a financial conflict of interest management plan, the nonFederal entity shall, within 120 days of the non-Federal entity's determination of noncompliance, complete a retrospective review of the Investigator's activities and the project funded under the DOE award to determine whether any project activity, or portion thereof, conducted during the time period of the noncompliance, was biased in the purpose, design, conduct, or reporting of such project.
ii. The non-Federal entity is required to document the retrospective review; such documentation shall include, but not necessarily be limited to, all of the following key elements:
1. DOE award number;
2. Project title;
3. PI or contact PI if a multiple PI model is used;
4. Name of the Investigator with the FCOI;
5. Name of the entity with which the Investigator has a financial conflict of interest;
6. Reason(s) for the retrospective review;
7. Detailed methodology used for the retrospective review (e.g., methodology of the review process, composition of the review panel, documents reviewed);
8. Findings of the review; and
9. Conclusions of the review.
iii. Based on the results of the retrospective review, if appropriate, the non-Federal entity shall update the previously submitted FCOI report, specifying the actions that will be taken to manage the financial conflict of interest going forward. If bias is found, the non-Federal entity is required to notify the DOE program office promptly and submit a mitigation report to the DOE program office. The mitigation report must include, at a minimum, the key elements documented in the retrospective review above, a description of the impact of the bias on the project, and the non-Federal entity’s plan of action or actions taken to eliminate or mitigate the effect of the bias (e.g., impact on the project; extent of harm done, including any qualitative and quantitative data to support any actual or future harm; analysis of whether the project is salvageable). Thereafter, the non-Federal entity will submit FCOI reports annually, as specified elsewhere in this Policy. DOE program offices may, by language in Funding Opportunity Announcements (FOAs) or by term and condition of award, require more frequent reporting for awards. Depending on the nature of the financial conflict of interest, a non-Federal entity may determine that additional interim measures are necessary with regard to the Investigator's participation in the project funded under the DOE award between the date that the conflict of interest or the Investigator's noncompliance is determined and the completion of the non-Federal entity's retrospective review.
5. Prior to the non-Federal entity's expenditure of any funds under a DOE award, the non-Federal entity shall ensure public accessibility, via a written response to a request within five business days of a request, of information concerning any significant financial interest disclosed to the non-Federal entity that meets the following three criteria
a. The significant financial interest is still held by the senior/key personnel as defined by this Policy;
b. The non-Federal entity determined that the significant financial interest is related to the project funded under the DOE award; and
c. The non-Federal entity determined that the significant financial interest is a financial conflict of interest.
d. The information that the non-Federal entity makes available via a written response shall include the following: the Investigator’s name; the Investigator’s title and role with respect to the project; the name of the entity in which the significant financial interest is held; the nature of the significant financial interest; and the approximate dollar value of the significant financial interest (dollar ranges are permissible: $0-$4,999; $5,000-$9,999; $10,000-$19,999; amounts between $20,000-$100,000 by increments of $20,000; amounts above $100,000 by increments of $50,000), or a statement that the interest is one whose value cannot be readily determined through reference to public prices or other reasonable measures of fair market value. The non-Federal entity will note in its written response that the information provided is current as of the date of the correspondence and is subject to updates, on at least an annual basis and within sixty days of the non-Federal entity’s identification of a new financial conflict of interest, which should be requested subsequently by the requestor. Information concerning the significant financial interests of an individual subject to the above paragraph of this section shall remain available for at least three years from the date that the information was most recently updated.
6. Reporting of financial conflicts of interest
a. Prior to the non-Federal entity’s expenditure of any funds under a DOE-funded project, the non-Federal entity shall provide to the DOE program office a FCOI report regarding any Investigator's unmanaged or unmanageable significant financial interest found by the non-Federal entity to be conflicting. DOE program offices may, by language in FOAs or term and condition of award, require a non-Federal entity’s FCOI report also list any Investigator’s significant financial interest found by the non-Federal entity to be conflicting and addressed by a management plan in accordance with this Policy. In cases in which the non-Federal entity identifies a financial conflict of interest and eliminates it prior to the expenditure of DOE-awarded funds, the non-Federal entity need not submit a FCOI report to the DOE program office.
b. For any significant financial interest that the non-Federal entity identifies as conflicting subsequent to the non-Federal entity’s initial FCOI report during an ongoing project funded under a DOE award (e.g., upon the participation of an Investigator who is new to the project), the non-Federal entity shall:
i. This clause is ONLY applicable when a DOE program office requires the non-Federal entity to include only unmanaged or unmanageable Investigator FCOIs in the FCOI Report:
1. Provide to DOE within sixty days an FCOI report regarding the financial conflict of interest if the non-Federal entity’s designated official determines that the FCOI is unmanageable. Pursuant to Paragraph 5(4) of this Policy, where such FCOI report involves a significant financial interest that was not disclosed timely by an Investigator or, for whatever reason, was not previously reviewed or managed by the non-Federal entity (e.g., was not timely reviewed or reported by a subrecipient), the non-Federal entity also is required to complete a retrospective review to determine whether any project funded under a DOE award or portion thereof conducted prior to the identification of the financial conflict of interest was biased in the purpose, design, conduct, or reporting of such project. Additionally, pursuant to paragraph (a)(3)(iii) of this section, if bias is found, the non-Federal entity is required to notify the DOE program office promptly and submit a mitigation report to the DOE program office.
ii. Applicable when a DOE program office requires the non-Federal entity to include all Investigator FCOIs – including managed and unmanaged/unmanageable FCOIs – in the FCOI Report
1. Provide to DOE within sixty days, an FCOI report regarding the financial conflict of interest and ensure that the non-Federal entity has implemented a management plan in accordance with this Policy. Pursuant to paragraph (a)(3)(ii) of this section, where such FCOI report involves a significant financial interest that was not disclosed timely by an Investigator or, for whatever reason, was not previously reviewed or managed by the non-Federal entity (e.g., was not timely reviewed or reported by a subrecipient), the non-Federal entity also is required to complete a retrospective review to determine whether any project funded under a DOE award, or portion thereof, conducted prior to the identification and management of the financial conflict of interest was biased in the purpose, design, conduct, or reporting of such project. Additionally, pursuant to paragraph 5(4)(iii) of this Policy, if bias is found, the non-Federal entity is required to notify the DOE program office promptly and submit a mitigation report to the DOE program office.
c. Any FCOI report required under Sections 6(b)(1) or 6(b)(2) of this section shall include sufficient information to enable DOE to understand the nature and extent of the financial conflict, and to assess the appropriateness of the non-Federal entity’s management plan. Elements of the FCOI report shall include, but are not necessarily limited to the following:
i. DOE award number;
ii. PI or Contact PI if a multiple PI model is used;
iii. Name of the Investigator with the financial conflict of interest;
iv. Name of the entity with which the Investigator has a financial conflict of interest;
v. Nature of any applicable financial interest (e.g., equity, consulting fee, travel reimbursement, honorarium) and/or applicable external relationships or activities;
vi. Value of any applicable financial interest (dollar ranges are permissible: $0 -$4,999; $5,000-$9,999; $10,000-$19,999; amounts between $20,000-$100,000 by increments of $20,000; amounts above $100,000 by increments of $50,000), or a statement that the interest is one whose value cannot be readily determined through reference to public prices or other reasonable measures of fair market value;
vii. A description of how the financial interest relates to the project funded under a DOE award and the basis for the non-Federal entity’s determination that there is a conflict with such project; and
viii. This section is ONLY applicable when a DOE program office requires the non-Federal entity to include all Investigator FCOIs – including managed and unmanaged/unmanageable FCOIs – in the FCOI Report:
1. A description of the key elements of the non-Federal entity’s management plan, including:
a. Role and principal duties of the conflicted Investigator in the project;
b. Conditions of the management plan;
c. How the management plan is designed to safeguard objectivity in the project;
d. Confirmation of the Investigator's agreement to the management plan;
e. How the management plan will be monitored to ensure Investigator compliance; and
f. Other information as needed.
d. For any financial conflict of interest previously reported by the non-Federal entity with regard to an ongoing project funded under a DOE award, the non-Federal entity shall provide DOE with an annual FCOI report that addresses the status of the financial conflict of interest and, if applicable, any changes to the management plan for the duration of the DOE award. The annual FCOI report shall specify whether the financial conflict is still being managed or if it remains Alternatively, the annual FCOI report shall explain why the financial conflict no longer exists. The non-Federal entity shall provide annual FCOI reports to DOE for the duration of the project period (including extensions with or without funds) in the time and manner required by term and condition of award.
e. In addition to the annual FCOI report, DOE may require a non-Federal entity to routinely submit all or some Investigator disclosures of financial interests. Circumstances when DOE may require a non-Federal entity to submit all or some of such Investigator disclosures include but are not limited to:
i. As part of monitoring the non-Federal entity’s compliance with this Policy;
ii. Bankruptcy of the non-Federal entity;
iii. Other legal winding down of the non-Federal entity;
iv. Acquisition of the non-Federal entity by a foreign entity, where “acquisition” includes a foreign entity obtaining a controlling interest in the non-Federal entity; or
v. As otherwise set forth in 2 CFR 200, as amended by 2 CFR 910.
f.
7. The non-Federal entity shall make initial and ongoing FCOI reports and supporting documentation to DOE as requested. The non-Federal entity shall maintain records relating to all Investigator disclosures of financial interests and the non-Federal entity’s review of and response to such disclosures (whether or not a disclosure resulted in the non-Federal entity's determination of a financial conflict of interest) and all actions under the non-Federal entity’s policy or retrospective review, if applicable, for the time period specified in 2 CFR 200.334 or, where applicable, from other dates specified in the individual award terms and conditions.
8. Whenever a non-Federal entity implements a management plan pursuant to this Policy, the non-Federal entity shall monitor Investigator compliance with the management plan on an ongoing basis until the completion of the DOE award.
9. The non-Federal entity shall establish adequate enforcement mechanisms and provide for administrative actions to ensure Investigator compliance as appropriate.
10. If the non-Federal entity carries out the DOE award through or with the assistance of one or more subrecipient(s), the recipient non-Federal entity must take reasonable steps to ensure that each subrecipient Investigator complies with this Policy by:
a. Incorporating as part of a written agreement with the subrecipient terms that establish whether the financial conflict of interest policy of the recipient non-Federal entity or that of the subrecipient will apply to the subrecipient's Investigators.
i. If the subrecipient's Investigators must comply with the subrecipient's financial conflict of interest policy, the subrecipient shall certify as part of the agreement referenced above that its policy complies with their own DOE Interim COI Policy and the subrecipient shall make such policy available via a publicly accessible website. If the subrecipient does not have any current presence on a publicly accessible website (and only in those cases), the subrecipient shall make its written policy available to any requestor within five business days of a request. If the subrecipient cannot provide such certification, the agreement shall state that subrecipient Investigators are subject to the financial conflict of interest policy of the recipient non-Federal entity for disclosing financial conflicts of interest;
ii. Additionally, if the subrecipient's Investigators must comply with the subrecipient's financial conflict of interest policy, the agreement referenced above shall specify time period(s) for the subrecipient to report all identified financial conflicts of interest to the recipient non-Federal entity. Such time period(s) shall be sufficient to enable the recipient non-Federal entity to provide timely FCOI reports, as necessary, to DOE, as required by this DOE COI Policy;
iii. Alternatively, if the subrecipient’s Investigators must comply with the recipient non-Federal entity’s financial conflict of interest policy, the agreement referenced above shall specify time period(s) for the subrecipient to submit all Investigator disclosures of significant
financial interests to the recipient non-Federal entity. Such time period(s) shall be sufficient to enable the recipient non-Federal entity to comply timely with its review, management, and reporting obligations under this Policy.
b. Providing FCOI reports to the DOE program office regarding all financial conflicts of interest of all subrecipient Investigators consistent with this Policy, i.e., prior to the expenditure of funds and within sixty days of any subsequently identified FCOI.