OKLAHOMA CITY, OKLA. – An innovative new field of research holds the promise for pediatric cardiologists and heart surgeons to predict the future structural integrity of a child’s heart valves so they can perform the best possible surgery today.
At the University of Oklahoma, a pediatric heart surgeon and cardiologist are collaborating with a biomedical engineer in a type of research that few in the nation are doing. By leveraging the expertise and technology of their respective fields, they are creating computational models to help them more fully understand the intricacies of individual hearts. Whereas traditional imaging techniques like echocardiogram provide a picture of the heart in motion, a computational model offers a simulated view of the shape of the valves, possible weak spots, the blood moving through the valves, and potential surgical steps to prevent future problems.
“This is truly translational medicine,” said OU Health pediatric heart surgeon Harold Burkhart, M.D., who is also a professor and chief of cardiovascular and thoracic surgery in the OU College of Medicine.
“Because of our multidisciplinary collaboration, we have the knowledge together to create a computational model that goes beyond what we are able to see with both 2D and 3D echocardiogram. It allows us to go a step further and visualize the heart as it would be in real life with the characteristics of each individual,” Burkhart said. “With that understanding, we can test what would happen if we put a stitch here or tighten a valve there — does it put too much stress on the valve, or does it address the problem? It can potentially give us a lot more direction before we even go into the operating room.”
The research group began their studies with hypoplastic left heart syndrome, a severe birth defect in which the left side of a baby’s heart does not form correctly and cannot pump blood well, leaving the right side of the heart to pump blood to the lungs and the rest of the body. Without a set of three of open-heart surgeries to repair the defect, the condition is often fatal. While continuing those studies, they are now developing computational models for atrioventricular canal defect, sometimes called a hole in the heart, because the baby is born with a hole in the wall separating the heart’s chambers.

While surgeries for both conditions are often successful, children sometimes face a risk of reoperation in the future because of leaky valves, Burkhart said. In hypoplastic left heart syndrome, about 25% of patients have leaky valves by the time they are in preschool. Babies with atrioventricular canal defect sometimes experience valve leakage six months to two years after the initial surgical repair, for reasons that are not yet understood. Follow-up surgeries are about half as successful as the original procedure, and if the repair can’t be made, the child may need a mechanical heart valve, which comes with its own set of risks and complications.
During surgery, when the heart is stopped and on a bypass machine, it lays flat and doesn’t maintain its structure as it would if it were pumping blood. Surgeons employ techniques to inflate the valves to improve their view, but heart surgery is time-limited and allows for no trial and error. If a computational model can predict which areas of the heart valves could become problematic, surgeons may be able to take steps to prevent that leakage in the future.
“Even with 3D echocardiogram, it’s hard to see the finer details of the valves,” said OU Health cardiologist Arshid Mir, M.D., an associate professor of pediatric cardiology in the OU College of Medicine. “Most heart valves are like Pringle chips — they are more angular and have high and low points. We want to learn about these valves and try to predict which valves, right at birth, would have a risk of becoming leaky when the child is between 1 and 5 years old, so that we can try to address them surgically at the time of the first repair.
“What we are doing is moving toward personalized medicine,” Mir added. “How do we think about each patient differently rather than thinking of a condition as a single disease? What is unique about this child’s anatomy that makes him at higher risk for reoperations? This is the way we will be thinking about these diseases as we head into the future.”

Burkhart and Mir work with Chung-Hao Lee, Ph.D., a former OU biomedical engineering researcher now at the University of California, Riverside. He builds computational models that draw from the fields of physics, fluid dynamics, geometry and bioengineering to create something that hasn’t existed before. Burkhart and Mir provide 2D and 3D echocardiograms of patients enrolled in the studies for the calibration of the models and for model prediction. They will continue to gather data from patients at multiple time points over the next several years.
“Through this image-based computational model, we want to provide the missing information — which patients will have valve dysfunction,” Lee said. “If we can better understand which valves have weak spots and may start leaking, surgeons may plan their surgeries differently and cardiologists may want to monitor or follow the patient more closely after surgery.”
Thus far, the research team has published about 10 papers on their computational modeling for hypoplastic left heart syndrome and recently published a paper on atrioventricular canal defect in the Annals of Biomedical Engineering.
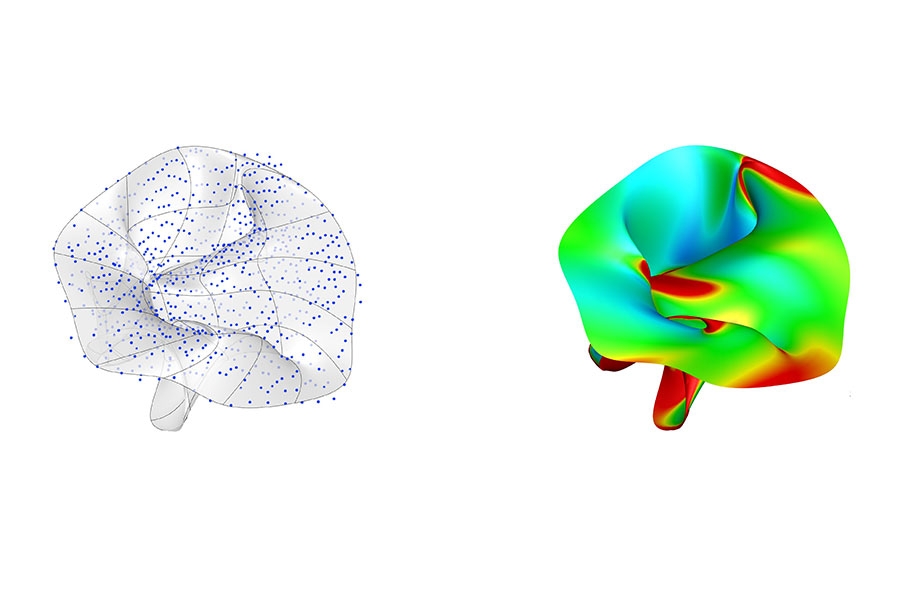
These images represent the data used in construction of a computational model (left) and the resulting prediction of potential heart valve leakage (right).
About the project
The research team’s latest paper, “Bayesian Optimization-Based Inverse Finite Element Analysis for Atrioventricular Heart Valves,” is at https://doi.org/10.1007/s10439-023-03408-6.
The research has been supported by the Presbyterian Health Foundation in Oklahoma City, the American Heart Association, the Oklahoma Center for the Advancement of Science and Technology, and the National Institutes of Health.
About the University of Oklahoma
Founded in 1890, the University of Oklahoma is a public research university located in Norman, Oklahoma. As the state’s flagship university, OU serves the educational, cultural, economic and health care needs of the state, region and nation. OU was named the state’s highest-ranking university in U.S. News & World Report’s most recent Best Colleges list. For more information about the university, visit ou.edu.
To combat power outages and extreme weather events, a team led by University of Oklahoma researchers has helped launch a project utilizing electric school buses as a backup energy resource.
The University of Oklahoma Health Campus was recently recognized for its increased momentum in advancing discoveries that change lives, achieving the state’s first Top 100 national ranking based on funding from the National Institutes of Health, according to the Blue Ridge Institute for Medical Research. The ranking—the highest in OU’s history and in the state—solidifies the University’s position as the state’s leading driver of health-related research.
Stefano Tarantini, an assistant professor in the Department of Neurosurgery at the University of Oklahoma College of Medicine, spends his days in the laboratory searching for answers to the cognitive decline that too often plagues older adults.